Our perspectives
in one place
Topic
Selected filters
ClearNews
See allPharmaceutical industry issues call for collective action to address the rising global burden of chronic disease
Read moreStatement delivered at the 13th Intergovernmental Negotiating Body (INB 13)
On 17 February 2025, IFPMA delivered a statement during the 13th Intergovernmental Negotiating Body (INB 13).
Read more156th WHO Executive Board (EB156): Constituency statement on climate change and health
On 8 February 2025 at the EB156 in Geneva, the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), Global Diagnostic Imaging, Healthcare IT & Radiation Therapy Trade Association (DITTA), Global Self-Care Federation (GSCF), and International Hospital Federation (IHF) delivered a statement on Agenda Item 22: Climate change and health.
Read more156th WHO Executive Board (EB156): Fight the Fakes Alliance statement on substandard and falsified products
On 7 February 2025 at the EB156 in Geneva, the Fight the Fakes Alliance delivered a statement on substandard and falsified medical products. IFPMA is a member of Fight the Fakes Alliance.
Read more156th WHO Executive Board (EB156): Constituency statement on health emergencies
On 6 February at the EB156, the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), Global Diagnostic Imaging, Healthcare IT & Radiation Therapy Trade Association (DITTA), Global Self-Care Federation (GSCF), International Pharmaceutical Federation (FIP), International Alliance of Patients’ Organizations (IAPO), and International Generic and Biosimilar Medicines Association (IGBA) delivered a statement on Agenda Item 15: WHO’s work in health emergencies.
Read more156th WHO Executive Board (EB156): Individual statement on non-communicable diseases
On 5 February at the EB156 in Geneva, IFPMA delivered a statement on non-communicable diseases (NCDs).
Read morePublications
See allTrade and Health Agenda: A Meaningful World Trade Organization Roadmap to Strengthen Global Health
Ahead of the 14th WTO Ministerial Conference (MC14), trade associations representing the pharmaceutical industry provide a perspective on how WTO Members can a roadmap to strengthen global health through meaningful trade policies.
Read more
Value of investment on NCDs: Impact on health outcomes in low- and middle-income countries
In new research commissioned by IFPMA, Airfinity has determined that an additional investment of 1% of GDP toward domestic general government health expenditure in low- and middle-income countries (LMICs) would amount to a US$ 310 billion additional investment, representing a 33% increase in current spending. This research is a response to the UHC 2030 action agenda...
Read more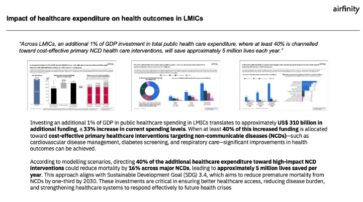
From innovation to access: Pharmaceutical industry priorities ahead of the 4th UN High-Level Meeting on NCDs and Mental Health
Non-communicable diseases (NCDs), such as diabetes, cardiovascular, renal and metabolic diseases, cancer, chronic respiratory diseases, mental health and neurological diseases, are one of the greatest global health and economic threats we face in our lifetime. Since 2000, global deaths due to NCDs have increased rapidly, even as deaths due to communicable diseases have declined. The...
Read more
Expert insights
See all
Strong regulatory systems support the pharmaceutical industry in delivering better health outcomes
Read more
Adaptive HTA: striking the right balance between rapid assessments and local health impact
Read more
Unlocking the full value of life-course immunization: A key to sustainable healthcare
Read moreResources
See allOur Ethos in Action – Decision-Making Framework Toolkit
IFPMA has developed a Five-Phase Decision-Making Framework, grounded in the IFPMA Ethos or value system, to help companies make decisions that balance business objectives and ethical considerations to meet patient needs and the expectations of the medical community, regulators, and society.
Read more
February 2024: Impact of a waiver of intellectual property rights for COVID-19 therapeutics
As discussions on an extension of a waiver of intellectual property (IP) rights on COVID-19 therapeutics continue, latest evidence and data published today explains what the adverse impact of a waiver may be on the entire innovation ecosystem and the consequences it may have on industry’s ability to fight future pandemics.
Read more
Action on NCDs: How the innovative pharmaceutical industry helps bridge the care gap
Read more



