How different partnership models supported the response against COVID-19

Date
7 June 2023
Time
11:00 - 12:30 CET, followed by lunch
Hosts
IFPMA
Location
Restaurant Vieux-Bois, Geneva & online
Attendance
This event has now passed. To browse our upcoming events click here.
At the outset of the pandemic, the life sciences industry made addressing the pandemic its top priority, devoting its resources, expertise, know-how, and intellectual assets to developing potential treatments, diagnostics and vaccines and committed to work in partnership with others at a record pace.
The unprecedented nature and scale of the pandemic affirmed the importance of partnerships to scale up manufacturing, without compromising safety and quality. These collaborations were voluntary, varied and dynamic, requiring close working relationships amongst partners. How was this achieved?
At the same time, the pandemic also highlighted bottlenecks. It heightened misperceptions about the complexity of these collaborations, including those relating to technology transfers and licensing models. Which lessons can be extracted from COVID-19 that can make these partnerships stronger, to be better prepared for future pandemics?
On 7 June, IFPMA hosted a Geneva Pharma Forum, where partners involved in these collaborations shared their experience and discussed the elements and frameworks that foster them.
Missed the event? Rewatch the recording below.
The discussion was moderated by Greg Perry, Assistant Director General, IFPMA.
The agenda and speakers are listed below (in alphabetical order).
Program
- Marco Alemán, Assistant Director General, WIPO
- Antony Taubman, Director, Intellectual Property Division, WTO Secretariat
- Thomas Cueni, Director General, IFPMA
- Rasmus Bech Hansen, CEO and Founder, Airfinity
- Tiwadayo Braimoh, Policy Advocacy, Medicines Patent Pool
- Leisha Daly, Head of Government Affairs & Policy EMEA Supply Chain Initiatives & Campus at Johnson & Johnson
- Osman Khalid Waheed, CEO of Ferozsons Laboratories Limited
- Marco Krieger, Vice-president of Health Production and Innovation in Health, Fiocruz
- Morena Makhoana, CEO, Biovac
- Kilian Mullet, Senior Director, Business Development, Pfizer
- Stavros Nicolaou, Group Senior Executive, Strategic Trade at Aspen Pharma Group
- Paula Pohja-Hutchison, Senior Director, Global Policy & Advocacy, AstraZeneca
- Hemal Shah, Director for Public Policy, Gilead
- Vignesh Shivnath, Business Development & Alliance Management, Dr Reddy’s Laboratories
- Julia Spencer, AVP, Global Multilateral Engagement, Strategic Alliances, and International Relations, MSD
Speakers

Marco Alemán is Assistant Director-General, IP and Innovation Ecosystems Sector at the World Intellectual Property Organization (WIPO), where he is responsible for helping Member States develop their IP and innovation ecosystems to drive economic growth. He is also responsible for providing support for researchers, innovators, and enterprises, including SMEs. Other key focus areas include IP commercialization for business growth; emergence of IP as an asset class; development of advisory expertise on national IP strategies; economic analysis on the role IP plays in promoting innovation and creativity; strengthening ADR and the services provided by the Arbitration and Mediation Center; and providing technical assistance to the judiciary, as well as services related to the legal databases. He joined WIPO in 1999.
Prior to joining WIPO, Dr. Alemán practiced as an IP Attorney and was then appointed Head of the Colombian Industrial Property Office.

Tiwadayo Braimoh has 22 years of work experience split evenly between the pharmaceutical industry and the non-profit sector. He currently works with Medicines Patent Pool (MPP), Geneva, Switzerlandd where he leads the policy advocacy aspects of the application of voluntary licensing and technology transfer to facilitate access to COVID-19 antivirals and products of pandemic relevance.
He started off his career in the pharmaceutical industry as a medical representative and proceeded to assume progressive roles in marketing and business development. Subsequently, he joined Clinton Health Access Initiative (CHAI) where he managed large-scale public health programs to improve access to medicines and worked with governments in Africa on health policy and health systems strengthening initiatives.
Mr. Tiwadayo Braimoh, a Nigerian, holds an MSc in Health Policy, Planning and Financing from London School of Economics and Political Science and London School of Hygiene and Tropical Medicine and an MBA from the University of Lagos. He is also a pharmacist with additional trainings in intellectual property and a Fellow of the West African Postgraduate College of Pharmacists.

Thomas Cueni is Director General at the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) and is Secretary of the global Biopharmaceutical CEO Roundtable (BCR).
Thomas has led the Association since 2017, during which time he has been instrumental in establishing cross sectoral programmes designed to tackle the biggest global health challenges. This includes establishing the $1bn AMR Action Fund to support the development of new antibiotics; as Chair of the AMR Alliance; as a member of the Board of City Cancer Challenge; and as the industry’s representative on the Access to COVID-19 Tools (ACT) Accelerator. Leading the IFPMA through the COVID-19 pandemic, Thomas galvanised the industry to develop the Berlin Declaration – the pharmaceutical industry’s blueprint to deliver equitable access to vaccines, treatments and diagnostics in future pandemics. Thomas is Chair of the Business at OECD Health Committee and serves as Industry Co-Chair of the APEC Biopharmaceutical Working Group on Ethics.
Prior to joining IFPMA, Thomas was Secretary General of Interpharma, the trade association representing Switzerland’s research-based pharmaceutical industry, Secretary of the Dolder Group, precursor of what’s now the BCR, and served on the Board of the European Federation of Pharmaceutical Industries and Associations (EFPIA). Thomas began his career as a journalist, as London correspondent for the Basler Zeitung and Der Bund, before serving as a Swiss diplomat in Paris (OECD) and Vienna (IAEA, UNIDO).
Thomas has a master’s degree in economics from the University of Basel and another in politics from the London School of Economics. He also studied at the Geneva Graduate Institute for International Studies.

Leisha Daly started her 23 -year career at J&J as Head of Medical Affairs at Janssen, and led in several senior positions in medical, sales and marketing management. In her role as Country Director, Dr. Daly successfully led Janssen through the economic downturn to become the fastest growing pharmaceutical company and the 5th largest pharmaceutical company in Ireland. Currently she is the Head of Government Affairs EMEA Supply Chain Initiatives and Campus.
Dr. Daly served as President of the Irish Pharmaceutical Healthcare Association (IPHA) from 2014-2016. In this role, she led industry talks with the State, and secured a pivotal four-year Framework Supply Agreement that offered stability in the supply of medicines to Irish patients and their treating clinicians. She is a member of the board of the American Chamber of Commerce Ireland and chairs their Sustainability Taskforce. Leisha is a member of Ibec’s National Council & chairs Ibec’s Energy & Climate Committee.
A strong advocate for the empowerment and advancement of women in the workforce, Dr. Daly is the J&J WiSTEM2D Ireland Programme Sponsor. This is a significant collaborative education programme in partnership with the University of Limerick, NUIG & UCC, that supports and encourages women to pursue educational opportunities in STEM subjects.
She was recognised as one of the Top 25 most powerful businesswomen in Ireland by the Women’s Executive Network in 2016 and in 2018 was recognised by the IMI with a Life Fellowship Award for her contribution to Irish Management.

Osman Khalid Waheed is the CEO of Ferozsons Laboratories Limited. He joined the company in 1993 after obtaining his undergraduate degree from Harvard University, USA. He has worked in logistics, sales and marketing before assuming the role of company president in 1999.
During this period, the company expanded its portfolio of medical solutions for critical diseases by forging alliances with a number of leading international partners including the Boston Scientific Inc., USA the world’s leading manufacturer of medical devices, a joint venture with the Bago Group of Argentina to establish BF Biosciences Limited, Pakistan’s first biotech pharmaceutical manufacturing company and Gilead Sciences, Inc. USA for their range of Hepatitis C and anti-viral therapies to name a few.
Mr. Waheed is currently serving on the boards of a number of companies including IGI Insurance, Lahore University of Management Sciences (LUMS), Murree Brewery, Nestle Pakistan Limited, and has previously served on the boards of Trade Development Authority of Pakistan (TDAP), Pakistan Industrial Development Corporation (PIDC), and as President of the Rawalpindi Chamber of Commerce and Industries.

Rasmus Hansen is a globally recognised expert on scientific information and knowledge dissemination. He spent his earlier career as a Fortune500 strategist. Rasmus is a strong believer in the power of accurate information and new ideas – he founded his first, short lived, newspaper at the age of 13 – a passion that drove him to found Airfinity. As CEO of Airfinity he is often quoted in leading media companies such as Financial times, Bloomberg and CNN. He is a board member of the newspaper group, Information.dk. Rasmus holds a B.Sc. in Political Science, a MPA from Harvard University and is a recipient of the Crown Prince Frederik award for excellent scholarship. He lives in London.


Morena Makhoana joined Biovac in 2004 and holds the role of Chief Executive Officer. He is a member of the Board and the Biovac Executive team. Prior to his CEO role at Biovac he held the role of Deputy CEO and prior to that of Medical Affairs Director for Biovac.
His mandate is to realise the objective of building vaccine manufacturing capacity in Southern Africa. Biovac’s vision and mission is to establish sustainable world class international African vaccine manufacturing capability by contributing to protecting life through the development, manufacture and supply of much needed and vaccines.
During his tenure Biovac has attracted three successful technology transfers with global pharmaceutical companies such as Sanofi and Pfizer that has allowed the company to grow its staff complement from 24 to over 450. Biovac’s next aim is to enter drug substance in order for Biovac and Africa to have end to end manufacture. Morena is a medical graduate from the University of Cape Town (UCT) in South Africa.
He has participated in numerous executive and business courses at both Harvard and Stanford Universities. He serves on a number of committees within the vaccine industry and serves as a board member of other healthcare and non-healthcare companies.

Kilian Mullet is a seasoned pharmaceutical professional and has worked in the industry since 1994 with Schering-Plough, Washington Group, Wyeth Biotech and is currently employed at Pfizer.
He graduated in industrial biology and holds an MBS in strategy and finance and a post graduate diploma in executive leadership.
In the early part of his career, he successfully led teams in green field pharmaceutical plant start-ups, capital project and operations management.
He progressed to be the global portfolio leader for Pfizer localisation programs, and he has enabled the technology transfer of vaccines and small molecules globally.
In his most recent role, he led the technical and supply aspects of Pfizer’s vaccines business development and all small and large molecule business development for Emerging markets and China.
He currently develops and enables commercial supply strategies for Pfizer’s equity, pandemic preparedness, and global product localisation programs.

Stavros Nicolaou is the Aspen Pharmacare Group’s Senior Executive responsible for Strategic Trade Development. Previously he was CEO of Aspen’s Export Business. Aspen is Africa’s largest pharmaceutical manufacturer and a now world leader in Anaesthetics and injectable anti-coagulants. Aspen is one of South Africa’s most globalised multinational companies with a presence in over 50 geographies globally, with 26 manufacturing facilities across 6 continents. He was instrumental in introducing the first generic ARV’s on the African Continent developed by Aspen, which has gone on to save hundreds of thousands of lives in South Africa and on the African Continent.
Dr. Nicolaou has over 28 years’ experience in the South African and International Pharmaceutical Industry and is a previous winner of the SA Institute of Marketing Management (IMM) Health Care Marketer of the year Award. He was inducted as one of the youngest Fellow of the Pharmaceutical Society of South Africa (PSSA), one of the highest honours bestowed by the PSSA, and was recently awarded an Honorary Doctorate of Science in Medicine from Wits University, the first pharmacist in the country to receive this.


Hemal Shah is director for public policy at Gilead Sciences, Inc., focusing on international intellectual property (IP), trade and supply chain policy. Most recently, she served as director for emerging markets at the U.S. Chamber of Commerce’s Global Innovation Policy Center (GIPC), focusing on India, Brazil, South Africa, and Russia. She also led the U.S.- India Business Council’s advocacy efforts in trade facilitation and supply chain policy.
Prior to her work with the U.S. Chamber of Commerce, Ms. Shah served as research associate for India and South Asia with the American Enterprise Institute (AEI) in Washington D.C. She previously managed the Legatum Institute’s global economic transitions program in London, was a scholar with the Takshashila Institution in Bangalore, and researcher with the United Nations Environment Program in Geneva. Ms. Shah recently served as visiting fellow (2019-20) with the German Marshall Fund (GMF), where her research focused on U.S.-India business relations. She has also served as a scholar with the GMF’s Young Strategists Forum in Tokyo, the Center for Strategic and International Studies’ Taiwan-U.S. Policy Program in Taipei, and the American Jewish Committee’s Project Interchange in Tel Aviv, with research focus on U.S. trade and security policy. Shah is the author of 40+ op-eds and research reports featured in publications including the Financial Times, the Economist, the Diplomat, and Foreign Policy.
Ms. Shah was recognized as “Emerging Leader 2018-19” by the Association of Women In International Trade (WIIT), a non-profit organization in Washington D.C., and volunteers as co-chair for WIIT’s healthcare priorities. She holds an MSc in Development Studies from the London School of Economics & Political Science and a BA in Business Management from the University of Nottingham, UK.

Vignesh Shivnath is Director, Business Development & Alliance Management at Dr Reddy’s Laboratories, India. He is manages External partnerships through Licensing, Co-Development and CDMO/CMO collaborations for Dr. Reddy’s.

Julia Spencer serves as the lead for MSD’s engagement with key global multilateral organizations and Geneva missions and is based in Geneva, Switzerland. She is also the focal point for and coordinates the company’s participation in international industry trade associations and business groups and directs the multilateral and strategic alliance policy agenda focused on UN health, trade, and innovation agencies; G7/G20 and other global government alliances; health security organizations; and economic and financing institutions. Prior to taking on this role, Julia was Associate Vice President, Global Vaccines Public Policy, Partnerships, and Government Affairs, during which time she led global policy and advocacy efforts to expand and sustain access to MSD’s vaccines and to strengthen the immunization systems delivering our products. Prior to joining MSD, Julia served for 15 years as a senior health official in the US Department of Health and Human Services (HHS), including as Science Policy Director within the HHS Secretary’s policy, planning, and evaluation office (ASPE), where she was responsible for policy coordination, planning, and legislative development focused on the HHS science agencies – Centers for Disease Control and Prevention, Food & Drug Administration, National Institutes of Health, and Office of the Assistant Secretary for Preparedness and Response.

Antony Taubman is Director of the WTO’s Intellectual Property, Government Procurement and Competition Division, with management responsibilities including coordination of trade and health. Formerly a diplomat, patent attorney in private practice and academic, he has served in senior policy roles in the Australian Federal Government and in the World Intellectual Property Organisation, and has taught, researched and published widely on IP law and policy issues.

Greg Perry is responsible for IFPMA’s Africa Engagement and Alliance Building strategies, focusing on innovation, access, and the regulatory environment. He has a long track record of leadership and advocacy in public healthcare and pharmaceuticals at international and European levels.
Before he joined IFPMA, Greg was Executive Director of the Geneva based Medicines Patent Pool. He was previously Director General of the European Generic Medicines Association in Brussels, which he started, and co-founder of the International Generic & Biosimilars Medicines Association (IGBA).
Greg also worked as the Managing Director and Partner in the Brussels office of a leading UK consultancy and as an advisor in the European parliament.
He has an MA in European Integration and Cooperation from the University of Hull, a BSoc.Sc in International Studies from the University of Birmingham, and a Diploma in Classical Studies from the Open University.
Greg is a Member of the Advisory Council of the Organization for Professionals in Regulatory Affairs (TOPRA) and is a former member of the Standing Advisory Committee before the European Patent Office (SACEPO).
He was awarded the Golden Cross of Merit of the Republic of Poland for his contribution to industry and European integration in 2004.
Resources
Applying Lessons Learned from COVID-19 to Create a Healthier, Safer, More Equitable World
This report identifies 10 insights the biopharmaceutical industry has gathered so far as the world moves into the third year of the COVID-19 pandemic.
Read more
Impact of a waiver of intellectual property rights for COVID-19 therapeutics
As WTO Member States continue to discuss an extension of a waiver of intellectual property (IP) rights on COVID-19 therapeutics, latest evidence and data published today explains what the consequences of such a waiver would have on industry’s ability to fight the COVID-19 pandemic.
Read more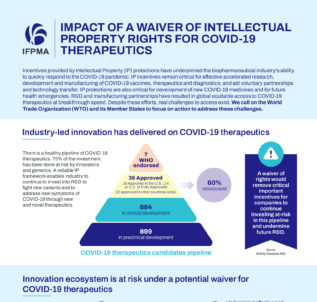
Technology Transfer: A Collaborative Approach to Improve Global Health
The transfer of advanced technology is essential for economic development. It enables countries to accelerate the acquisition of knowledge, experience and equipment related to advanced, innovative industrial products and processes. This, in turn, builds the skills of the local workforce and creates new high-tech employment opportunities.
Read more


