Statement
27 Aug 2025
AMATA statement at 75th WHO AFRO: Progress report on the regional strategy on regulation of medical products in African region
On 27 August 2025, the African Medicines Agency Treaty Alliance submitted a statement on agenda item 16.8: Progress report on the regional strategy on regulation of medical products in African region, 2016-2025 at the 75th Session of the WHO Regional Committee for Africa. The African Medicines Agency Treaty Alliance (AMATA) commends the progress made under...
Read more
Statement
27 Aug 2025
75th WHO Regional Committee for Africa: Progress report on the implementation of the strategy for scaling up health innovations in the African region
On 27 August 2025 in Lusaka, Zambia, IFPMA delivered a statement at the 75th session of the WHO Regional Committee for Africa focused on Agenda item 16.2: Progress report on the implementation of the strategy for scaling up health innovations in the African region. IFPMA takes note of WHO AFRO’s Progress report on the implementation...
Read more
Infographic
23 Jul 2025
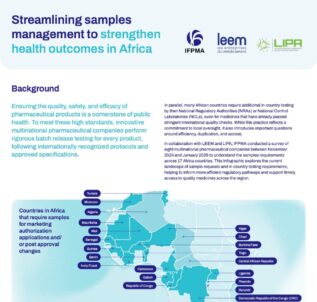
Streamlining samples management to strengthen health outcomes in Africa
Ensuring the quality, safety, and efficacy of pharmaceutical products is a cornerstone of public health. To meet these high standards, innovative multinational pharmaceutical companies perform rigorous batch release testing for every product, following internationally recognized protocols and approved specifications. However, countries may require additional in-country testing by their National Regulatory Authorities (NRAs) or National Control...
Read more